 |
Metals and Nonmetals
Order of Elements in the Periodic Table
Scientists arrange the elements in a table called the periodic table. So, what is the periodic table?
The Periodic Table: It consists of squares arranged in horizontal rows called periods and vertical columns called groups. Each square contains information about an element, including its name, chemical symbol, and the number of protons that distinguishes it from other elements.
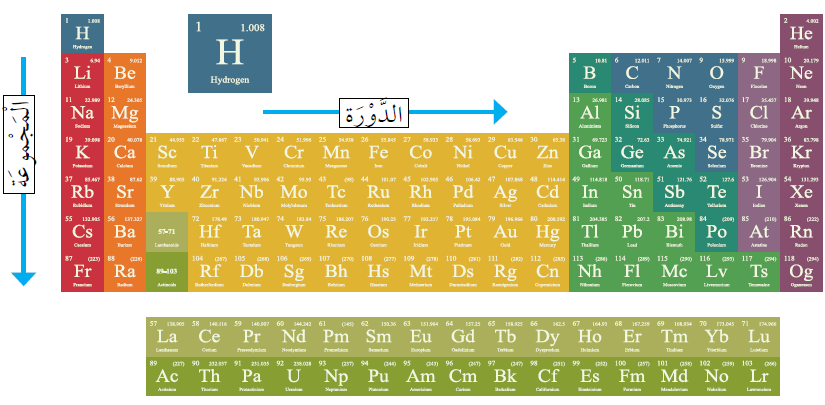
Elements in the same group have similar physical and chemical properties, and these properties repeat periodically in each period. That's why it's called the periodic table.
Metals and Their Properties
Metals are located on the left and middle of the periodic table (except for hydrogen).
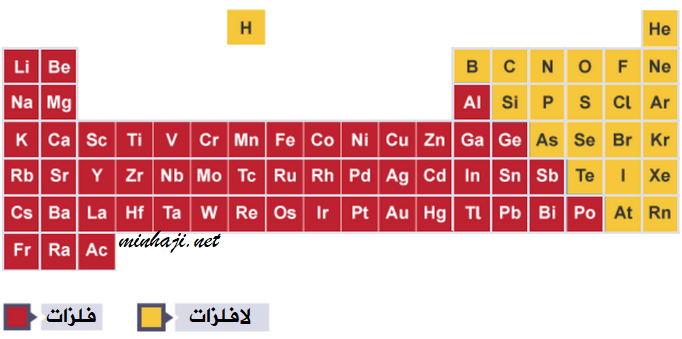
Properties of Metals:
- Metals are solid at room temperature (except for mercury, which is liquid).
- Metals are shiny.
- Metals are malleable and ductile, meaning they can be hammered into sheets or drawn into wires. For example, aluminum foil used for food packaging and copper wires.
- Metals are good conductors of heat. When you touch a metal spoon, it feels warm after stirring hot food. Metals vary in their ability to conduct heat, with aluminum and iron being good conductors. That's why they are used in cookware.
- Metals are good conductors of electricity. Metals can pass electric current in a closed electrical circuit. Metals also vary in their ability to conduct electricity, with copper and silver being good conductors. That's why copper is used in electrical wires.
Nonmetals and Their Properties
Nonmetals are located on the right side of the periodic table.

Properties of Nonmetals:
- Nonmetals can be solid, liquid, or gas at room temperature. For example:
- Phosphorus (P4) and iodine (I2) are solid.
- Bromine (Br2) is liquid.
- Most nonmetals are in the gas state, such as oxygen (O2) and nitrogen (N2).
- Nonmetals are not shiny.
- Nonmetals are not malleable or ductile. When nonmetals in solid form are struck, they crumble.
- Nonmetals are poor conductors of heat and electricity. Despite carbon being a nonmetal, it is a conductor of electricity.
Uses of Nonmetals:
- Phosphorus is used in the production of fertilizers and matchstick heads. It is also required by the human body in limited quantities and obtained from seafood, chicken, and nuts.
- Chlorine is used in water disinfection tablets and bleach.
Metalloids and Their Properties
There are elements that separate metals and nonmetals in the periodic table. These elements share common properties with both metals and nonmetals and are called metalloids.
Metalloids: They are a group of elements that share some properties with metals and others with nonmetals.
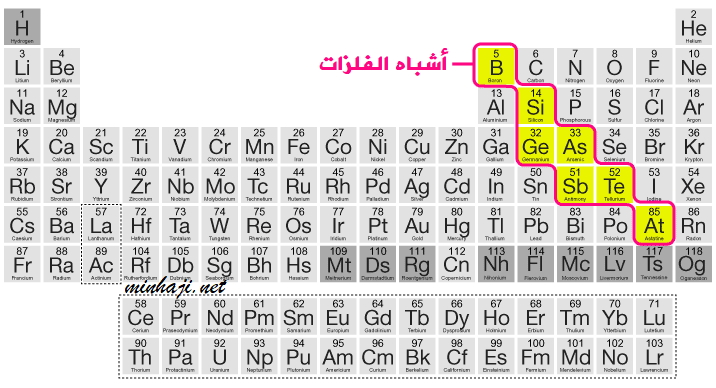
Metalloids are solid at room temperature. Silicon (Si) and germanium (Ge) are examples of metalloids. They are known for their ability to conduct electricity, so they are used in the production of electronic devices.